Before you start reading:
This article intentionally over-generalizes some scientific concepts for educational purposes. For those who wish to engage with these concepts more extensively, please click on the “Digging Deeper” buttons to learn more.
If you would like to skip to our section on how to use Sweetgum, click here!
The Story of "DIY Tamiflu®"
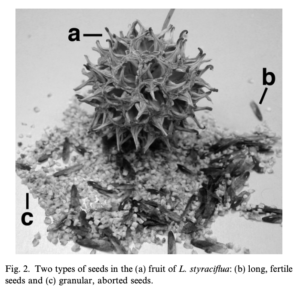
It is a widely held belief that the infertile seeds of Sweetgum (Liquidambar styraciflua) can be tinctured in alcohol to make an at-home Tamiflu® substitute [1].
This is because the infertile seeds contain ~6.5% w/w shikimic acid: the chemical used as the starting material for the synthesis of the antiviral drug Tamiflu®, which has been used to treat the early stages of Influenza A and B [2] [3].
Until 2005, Star anise (Illicium verum) seeds were one of the primary sources of this compound. Then, a shortage of them spurred researchers into a desperate search for alternative sources. Listed among these alternatives were the infertile seeds of the Sweetgum tree [3].
Because these infertile seeds contain shikimic acid–the chemical precursor to Tamiflu®–many people believe (or have been taught) that either:
- Shikimic acid (and, by extension, an extract of Sweetgum seeds) acts like Tamiflu® in the human body
- Or, shikimic acid is the active ingredient in Tamiflu®, not the starting material
Some of the extraction methods taught in these communities:
- Harvest the immature seed pods and allow them to dry in your car or in the oven, so that the infertile seeds fall out. Discard the now matured brown infructescence (seed pods), and collect the granular seeds. Tincture these in vodka (or Pure Grain Alcohol) for an unspecified amount of time.
- Harvest the green, immature seed pods of sweet gum. Cut them in half and pack them densely into a mason jar. Cover them with vodka (or Pure Grain Alcohol), seal the jar, and allow them to sit in a dark area for a while. You might notice the solution turning red or burgundy. After an unspecified amount of time, strain the solution and place it into an amber bottle.
- Decoct (boil) the green balls for an unspecified amount of time, and consume the resulting extract.
Figure 1, Infertile Seeds of Sweet Gum (c)
These are strong assumptions. If either of them are true, then the world has been missing out on the most incredible backyard medicine of the century. If false, then we’ve been duped.
So, what questions should we answer before we can know if these assumptions are valid or not? First, we need to know…
How does Tamiflu® work?
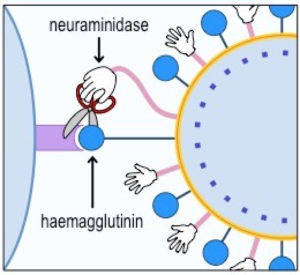
Oseltamivir, known by its commercial name “Tamiflu®,” is an antiviral drug used in the treatment of patients with Influenza A and B who have only been symptomatic for a couple days [4] [5] [6]. There are many kinds of antiviral drugs that target different aspects of viral disease, but this one works specifically as an inhibitor of the neuraminidase enzymes of Influenza A and B [4] [5] [6].
[For a visual explanation of what a neuraminidase inhibitor does, check out the video below!] [8]
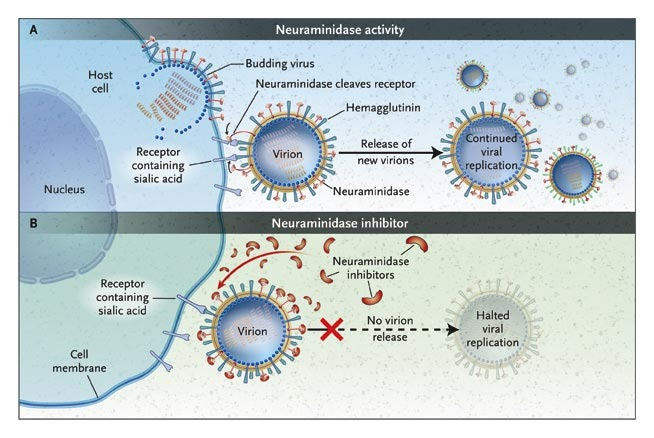
Let’s start with what a viral neuraminidase is. It is an enzyme–that is, a protein able to perform specific chemical reactions. Located on the surface of influenza, its job is to help the virus detach from an infected human cell by cleaving the bond between the cell and the viral protein hemagglutinin [7].
Figure 2, Actions of Neuraminidase [31]
This occurs after a cell has already been infected with the virus and when one of the newly made viruses is trying to leave the infected cell. Once the neuraminidase enzyme breaks this bond, the newborn virus is free to go infect other cells. If it can’t break the bond, then the virus never detaches from the infected cell and further infection is reduced significantly [7].
That’s where Tamiflu® comes in. It targets the neuraminidase enzymes of Influenza A and B by crawling into a little molecular pocket on the enzyme called the “active site” and preventing it from cleaving that cell-virus bond [7]. It is important that Tamiflu® fits properly in that pocket so that it can effectively “inhibit” the enzyme. So, the drug’s shape matters a lot. We sometimes call this “specificity.”
Figure 3, Visualizing a Neuraminidase Inhibitor
Speaking of shape, now let’s see how Tamiflu® snuggles up into this neuraminidase to lock it up and prevents it from allowing the new virus to escape!
Take a look at the image below [Figure 4]. This is the chemical “skeleton” of Tamiflu in a H5N1 (subtype of Influenza A) neuraminidase pocket (grey).
See how its structure fits the contour of the space?
Chemicals do not look like a bunch of balls and sticks. We represent them this way to understand their core structure and orientation in space. In reality, they have mass and take up space. Search google images for “molecular electron density electrostatic potential” to get a glimpse of the cool ways we model chemicals.
Figure 4, Tamiflu® in a Neuraminidase “Pocket”
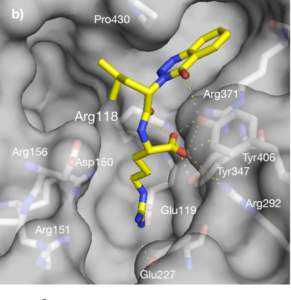
For simplicity’s sake, we can think of the relationship between the viral neuraminidase enzyme and Tamiflu® like a lock and key. They need to fit each other to work in a certain way.
The relationship between a viral neuraminidase and Tamiflu® can be more accurately described by a model called “induced fit.” This simply says that the shape of the enzyme changes in response to binding with a substrate (in this case, Tamiflu) [32] [33].
That is why the chemical structure of a drug matters so much. Form determines function. Structure is one of the most important parts of figuring out how a drug will act in our bodies, and how effectively it will do so [12] [13].
A wheel needs to be round, otherwise it would not be able to rotate and perform the “functions” of a wheel. Its structure is part of its identity and its purpose. Chemicals aren’t much different.
In fact, in molecular pharmacology, this “form determines function” principle is foundational to what is called “structure- or ligand-based approaches” to drug design–an exciting realm of research. In essence, it’s about designing drugs based on their targets–and, very powerful computers and programs play a role in identifying potential chemical structures that might work on a drug target (or, in our case, enzyme) of interest. Once candidate drug structures have been identified, chemists, pharmacists, biochemists and a host of other scientific professionals can get to work on making these drugs and testing their efficacy on the target [34]. For those who really want to take a deep dive into the form-is-function idea, Google “density functional theory.”
So, why does all of this matter?
Take a look at the chemical structure of shikimic acid below and compare it to the structure of Tamiflu®, do you think they fit into the neuraminidase pocket in the same way?
If your answer is “no,” then you’re right. There is no evidence to suggest that shikimic acid acts on neuraminidase enzymes and by looking at the two side-by-side, we can see why: shikimic acid is not going to fit into that neuraminidase “pocket” in the same or a similar way. It won’t stop that virus from flying off into intercellular space. It doesn’t act like Tamiflu®.
It might seem overly simplistic, but we can indeed conclude that the two chemicals do not act similarly just based on their structure–they are sufficiently different from each other.
It might seem like we’re just playing a molecular matching game, but the importance of structural similarity cannot be stressed enough.
“Structure” isn’t just about what a chemical looks like, it’s also about the functional groups (i.e. alcohols [R-OH], carboxylic acids [R–COOH], ethers [R–O–R′], thiols [R−SH], amino groups [-NH2], etc. etc. etc.) that define its reactivity, its solubility, and so much more. It is groups like these which determine if (or how well) a drug will interact with an enzyme (which also has its own “groups,” known as R-groups or residues in its active or allosteric sites that interact with molecules).
Shikimic acid and Tamiflu® do not act differently simply because they look different, but because their structures are defined by these chemical groups and these groups determine whether or not that molecule can “interact” with the chemical groups of the enzyme. Now, if we want to be good scientists, we would want to run an experiment to test whether or not the statement that these two do not act the same way (or similarly) on neuraminidases is actually true.
However, there are a couple reasons for why this is probably unnecessary. The first is that 1) it has not already been done. If shikimic acid was a viable substitute for Tamiflu®, do you really think we’d be spending all of this time making Tamiflu® from shikimic acid? No. There’s a reason why we use shikimic acid as the starting material, and it’s not because it acts similarly. And the second reason is 2) the structural differences between shikimic acid and Tamiflu® are significant enough to suggest that, on a theoretical level, we can refute the idea that they act similarly. The more similar two chemicals become in their structure, the more experimental evidence would be required to resolve their pharmacological similarities and differences.
This also means that compounds with only slight differences might act very similarly, which is another reason why Tamiflu® isn’t the only drug used to treat Influenza A or B. There are a host of other viral neuraminidase inhibitors, both synthetic and natural.)
Figure 5, Structural Comparison of Shikimic Acid & Tamiflu®
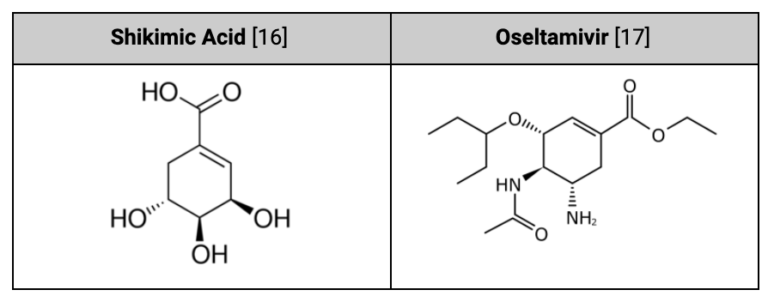
But based on its structure alone, can we say that shikimic acid is not antiviral?
No, we cannot claim that, but it is important to note that there is no scientific evidence whatsoever to suggest that it has any antiviral properties [35].
But, we can say that it is not antiviral in the same way Tamiflu® is antiviral due to its structural differences.
This brings us to an important point: not all antivirals are created equally. They are used to treat viral infections, but there are many ways to accomplish this: from highly specific drugs like Tamiflu® to more broad spectrum ones [18]. They can act on different parts of the virus or the host cell. Even immuno-modulating drugs are sometimes thrown into this category.
Figure 6, Types of Antivirals Based on their Targets
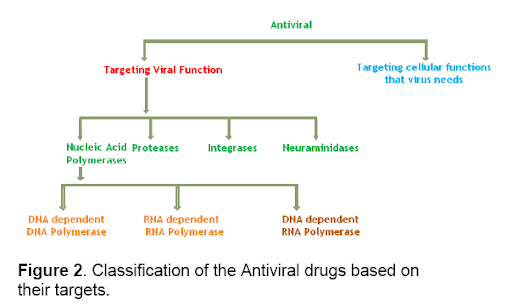
Lastly, structural specificity does not necessarily imply exclusivity. Tamiflu® is not the only compound that inhibits neuraminidase enzymes.
And, while shikimic acid is not a neuraminidase inhibitor, many other compounds from natural products are being investigated as potential viral neuraminidase inhibitors.
This was actually brought to our attention by Sam Coffman—a clinical herbalist and the founder of The Human Path and Herbal Medics Academy. If you want to learn more about the plants involved in this research, check out Appendix A at the end of the article. Thanks, Sam!
How is Tamiflu® made?
This section addresses the idea that shikimic acid is the active or even “main” ingredient in Tamiflu®.
In Figure 7, we see that shikimic acid is the starting material in the multi-step synthesis of Tamiflu® [19].
It is used as the starting material not because it has antiviral properties, but because it is a convenient structural building block for the synthetic process, thus reducing the amount of steps necessary and saving drug companies money [20].
Fun fact: there are actually many ways to synthesize Tamiflu®, and not all of them use shikimic acid [19].
Figure 7, Karpf / Trussardi Synthesis of Tamiflu®
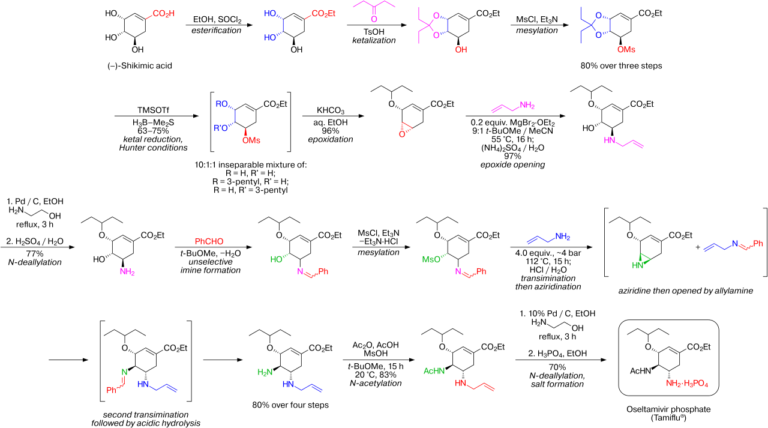
In chemical synthesis, the starting material is not necessarily the “main” (much less the “active”) ingredient. It is simply one step in a process. This goes against our typical intuition about mixing things up into something new. And, perhaps, these metaphors will help clarify shikimic acid’s actual place in Tamiflu®’s synthesis:
What Shikimic Acid is NOT
Let’s think about lettuce. When you make a salad, you combine lettuce (the “main” ingredient) with a variety of other items–a vinaigrette, tomatoes, cucumbers, maybe some cheese. But the lettuce is still lettuce, even if you built something new with it. It has the same nutritional value as it did before you added the other ingredients.
Shikimic acid doesn’t work like this. If you use it to build Tamiflu®, you aren’t making a salad, where shikimic acid is still acting like shikimic acid. You’re altering the identity of the lettuce itself to make this pharmaceutical “salad.”
What Shikimic Acid IS
Shikimic acid is like the frame of a car. On its own, the car frame cannot get you anywhere. But, when you build onto it–the engine, the wheels, and everything else–then it can take you places. You would never say that the car shares the same identity as the frame, this would be a fallacy (specifically, the Fallacy of Division) where you assume the characteristics of the whole are shared by that of the part [21].
In the same way, shikimic acid is used to make Tamiflu®, but that does not mean it is similar to Tamiflu®.
Shikimic Acid's Role in Nature
So, what’s the point of shikimic acid? Is it useful? Absolutely.
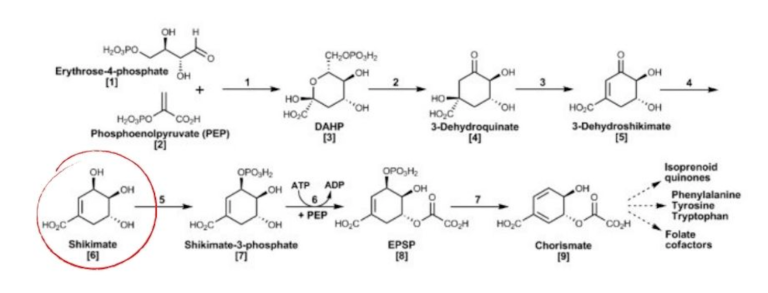
It plays a vital role in a critical metabolic pathway in plants and other non-mammalian organisms. This pathway provides the plant with folates, aromatic amino acids, and more–all necessary to its survival [23].
It is known as the “Shikimate Pathway,” and shikimic acid is one of the key stepping stones to these other essential compounds.
In the storage tissue of many seeds, shikimic acid will accumulate because many metabolic pathways have been shut off or slowed down. This is why we tend to see higher concentrations of it in seeds and also why we usually extract shikimic acid from the seeds of Star Anise and Sweetgum, not the rest of the plant [22].
Figure 8, The Shikimate Pathway
The Traditional Context of Sweetgum
When understanding the uses and efficacy of an herbal medicine, the two pillars of evidence typically referenced are either 1) scientific and/or 2) traditional knowledge.
We just figured out that:
- The shikimic acid in the infertile seeds of Sweetgum does not work like Tamiflu®
- There is currently no scientific evidence to suggest that shikimic acid functions as an antiviral (although there is evidence that it has antimicrobial properties!)
But, maybe the infertile seeds of Sweetgum–or, even the entire immature seed pods that contain them–are antiviral in another way?
To answer that, let’s turn to the second pillar of evidence: the traditional uses of the plant.
Sweetgum is a popular herbal medicine with an impressively long history of use.
We wanted to see if there were any accounts of the seeds pods (or any other part of the plant, for that matter) being used as an “antiviral” in traditional literature.
From the Native American Ethnobotany Database, we can search for a plant to get filtered results of its documented uses from these cultures. The page on Sweet Gum has 16 documented uses. Among these, not a single one of them mentions using the fruit [24]
Figure 9, search of “Liquidambar styraciflua” on the Native American Ethnobotany Database

Parts commonly used included the resin (“gum”), inner bark, and root. These documented uses come from the histories, traditions, and accounts of people who have lived on the North American continent, where Sweet Gum grows natively in some parts, for the longest period of time [24] [25].
Now, let’s turn to more recent accounts.
Tommie Bass (c. 1908-1996) is arguably the 20th century’s best known and most loved Southeastern traditional folk herbalist. What does he have to say about Sweetgum in A Reference Guide to Medicinal Plants?
Tommie tells us that he has indeed used it for coughs and colds. But, what parts did Tommie say he used for this? The leaves in the summertime, and the bark in the winter. [26]
There is one anecdotal reference that suggests he used ash from the fruits mixed with lard to help with skin conditions, but otherwise, he focused on the bark and leaves [36].
You’ll find that this is a common use listed in other references to the tree (see below).
Still nothing to confirm any efficacy of the seeds or seed pods specifically as antiviral agents…
Let’s expand our sources to the Eclectic doctors of North America. There are several, so they are just listed here:
- The Dispensatory of the United States of America, 1918 – Joseph P. Remington, Horatio C. Wood
- Specific Medication and Specific Medicines, 1870 – Scudder
- A Manual of Organic Materia medica and Pharmacognosy, 1917 – Sayre
- King’s American Dispensatory 1898 – Felter, Lloyd
- The Eclectic Materia Medica, Pharmacology and Therapeutics, 1922 – Felter
- The Physiomedical Dispensatory, 1869 – Cook
In all six of the books listed above, not one mentions the use of fruit or seeds for the treatment of flu-like illnesses.
Almost all of them do make note of Sweetgum’s efficacy in treating flu-like symptoms, but only from preparations of the root, bark, or resin–not the isolated infertile seeds or seed pods [27].
This supports what we found in Tommie’s accounts as well.
Right when we thought we had it figured out, “Lu Lu Tong” swooped in. Lu lu tong (meaning, “all roads open”) is the Chinese name of the American Sweetgum’s cousin species: Liquidambar formosana, or “Chinese Sweetgum.” Finally, some uses for the fruit! Yet, seemingly none of these uses were for viral infections or flu-like symptoms [28] [29].
A popular Traditional Chinese Medicine (TCM) Materia Medica says that the best time to process the fruit is when it is “yellowish brown” or brown, and this is consistent with the fruits we saw being sold on the internet under this name: the brown, seedless pods of Sweet Gum [37].
Figures 10 & 11, Images of “Lu lu tong” Herb from Online Suppliers [38]


This makes a lot of sense from an ethnobotanical perspective: of course they would use the dried, brown fruit… the fruit that has lost its granular, infertile seeds–this is what you typically find on the ground. It would be far less labor intensive to harvest them this way than the “modern” Southeastern way: by picking the green, not-yet-fallen fruit from hard-to-reach tree limbs.
We saw accounts saying that the extract moves Qi, expels wind, invigorates the blood, treats irregular menstrual cycles, promotes lactation, alleviates allergies, and more [37]. Just not anything for influenza-like illness.
(If you are reading this, and you do know that it is used to treat the flu in TCM, please share your references and experience in the comments below!)
Then, something really unexpected popped up: a single drug-discovery study from 2011 that surveyed 439 traditional Chinese remedies and, in doing so, found that a highly concentrated extract of Liquidambar formosana infructescence exhibited some neuraminidase inhibitory activity in vitro against a particular strain of H1N1 (another Influenza A subtype) [39].
Now this piqued our curiosity.
There are many reasons for why this article still does not validate the “DIY Tamiflu®” trend and why we should interpret its conclusions with skepticism, but it certainly shows that Nature can be quite serendipitous when she wants to be.
Click on “Digging Deeper” to learn more about this study & its implications.
- This the only experiment of its kind, and this use for the fruits of L. formosana has not been investigated further since this article was published.
- In order to confirm a finding like this, further exploration and experimentation would be necessary. For example, I would want to see in vitro studies one more types of influenza viruses, an in vivo study in mice, studies on isolating the constituents of L. formosana and identifying a list of potential active ingredients, drug-target kinetics studies, some high-quality crystallography, or (in a very ideal world) a clinical trial before really giving credence to the claims made in this article. A lack of follow-up does not invalidate a study, but it doesn’t do much to support its conclusions either. We should be cautious about taking “one of its kind” studies too seriously.
- The inhibitory effects were observed in vitro, not in vivo.
- In vitro means that the experiment was conducted outside of a living organism, whereas in vivo means that the experiment was conducted inside of a living organism (for example, clinical trials). The weakness of in vitro studies is that they cannot come close to simulating how a drug behaves in a living organism. To really confirm if a drug (or extract) has neuraminidase inhibitory capabilities, an in vivo study in mice should be conducted [40].
- The extract was most likely prepared using the dried, brown infructescence of L. formosana that lacked most of the fertile and infertile seeds.
- Their preparation of the L. formosana fruit is significantly different from the modern Southeastern method. We already know that the TCM preparation is with the dried brown fruits–the stage at which it has lost most of its seeds [41]. They boiled these in water, put it through centrifugation, filtered and freeze-dried the extract into powder before diluting it for experimentation. With only a few exceptions, the modern Southeastern method is to take the immature green fruit (or just the seeds themselves) and tincture them in alcohol before straining and storing. Do these different preparations bring out different kinds and amounts of constituents? Most likely, yes.
- Some of the other herbal extracts that the article claims have potential neuraminidase inhibiting activity (for example, Prunella vulgaris) were confirmed to be anti-viral, but not in the ways suggested by this study.
- This demonstrates the importance of “follow-up” and a body of literature. Prunella vulgaris was one of the most potent in vitro inhibitors in this paper (more potent than the L. formosana extract) , but it has an extensive amount of research behind it to suggest it has anti-viral properties, just not as an effective neuraminidase inhibitor. For example, in another drug-discovery/neuraminidase assay study from 2018, Prunella vulgaris was determined to be “not active” in its ability to inhibit a viral neuraminidase, with only 14.1% inhibition of the virus (in comparison to the pharmaceutical Zanamivir, which had 99.8% inhibition) [42]. This is exactly why we scientists need to follow-up on the activities of L. formosana extract before we can conclude that it is, in fact, an effective neuraminidase inhibitor.
- The authors clearly state that further investigation needs to be done to determine the mechanism of this potential neuraminidase inhibition as well as the active ingredient(s) responsible.
- The authors themselves state that more research needs to be done on this, and hope their paper is the reason this additional research is conducted.
- Liquidambar formosana is not the same as Liquidambar styraciflua (American Sweetgum), although they are closely related [43].
- There are many examples in herbalism where different species of the same genus have wildly different uses (Hypericum spp., for example). While sharing a genus might mean that two plants share a lot of characteristics, it is not a guarantee that they have the same kinds or levels of constituents.
This study does not prove (or even claim to prove) that the brown seed pods of Sweet Gum are a viable alternative to Tamiflu® .
In fact, as a drug-discovery study, its job is simply to give scientists a starting point for further investigation, and that’s exactly how we should take it too.
As we’ve already discussed, plants can produce compounds that function as viral neuraminidase inhibitors, and in a crazy coincidentally way, it turns out that one of the plants used to make a synthetic neuraminidase inhibitor (i.e. Tamiflu®) might contain a natural neuraminidase inhibitor of its own.
Does this mean we should all start boiling down the mature fruits of American Sweetgum and using the extract as an at-home remedy for the flu? That’s not really our place to say.
Does this study mean that all the misinformation being spread about making a “DIY Tamiflu®” by making an extract of the immature green fruits is actually… correct? Absolutely not.
Remember, the reason this myth came into existence was not because of some miraculous feat of pharmacological intuition. Instead, it was because a group of people misunderstood the relationship between shikimic acid and Tamiflu®–and, they tried to equivocate the two.
American Sweetgum balls might (a big MIGHT) contain a neuraminidase inhibitor that acts similarly to Tamiflu®–but that chemical is definitely not shikimic acid, and it’s probably not coming from the infertile seeds.
On the Nature of Evidence
Why did we go through all this trouble to search for ethnobotanical uses of the tree’s fruits when modern science had so little to say about their ability to treat viruses?
Because traditional ecological knowledge can give us incredible insights into medicinal reservoirs in nature that scientists have not yet had the time (or money or manpower) to investigate.
One person’s experience (otherwise known “expert opinion”) is one of the lowest forms of evidence [30]. But, an entire culture’s experience with a medicinal plant + a lot of time can potentially reveal useful patterns of healing for future research. So, we treat this type of experiential evidence with a bit more respect.
Yet, it is the “expert opinions” of a few individuals–mainly herbal folklore instructors, YouTubers, and bloggers–who incorrectly connected the dots and started the spread of this “DIY Tamiflu®” myth.
Let’s be very clear here, there is no judgement: these people were probably well-meaning, really excited to find a link between Sweetgum and a well-known pharmaceutical, and had platforms and audiences already set-up to receive this message.
In fact, that’s how we were taught the myth: in an online video. We never questioned what we were told about it, we just accepted the statement as true.
So, despite our backgrounds (Jesse is an electrical engineer by training, Laramie is majoring in plant bioinformatics & biotechnology), we also fell prey to this common misconception about Sweetgum and shikimic acid. If you did too, you’re not alone–join the club!
But, this brings us to an important point about the nature of personal testimony.
Many people in the Southeast will probably still say they have used a tincture of the Sweetgum fruit or isolated seeds and found that it works against the flu.
How should we respond to this kind of information?
We should be very cautious. Why? Mainly because there are countless ways in which this experience could have unknowingly been compromised:
- Did this person take only the Sweetgum extract when they had the flu? Or, was it part of a formula where other constituents could have contributed an effect?
- Were they actually battling the flu or something else? Is there a medical record?
- Have they compared their recovery with and without that particular medicine?
- Have they accounted for the placebo effect?
- And more…
Wait–so do we need to conduct expensive double-blind, placebo-controlled studies before making any claims about the efficacy of a particular herbal preparation?
No! But it wouldn’t hurt to take some notes and cite your sources.
At least leave some breadcrumbs so that people can follow the path of your logic, and maybe even try to replicate that experience for themselves!
This protects you, and this protects those who take your advice.
Articles That Spread the Myth
This section is not intended to call anyone out. We have had several people that have reached out to us asking if we have read article X or to ask “but this article says…”. This section is just to show that- yes, we have seen those articles and no, they do not present any information that has not already been addressed in this debunking.
Articles:
Traditional Uses Personal Experiments
To balance all the information about how Sweetgum can not be used, we thought it best to provide some experiments that investigate how it can!
We harvested and prepared several different components of sweetgum: inner bark, roots, leaves, and green gumballs. Next, we made decoctions of all of them individually and tried them for some sensory testing. This is what we found.
The decoction made from the root and inner bark was quite resinous and had a very dark color. Whereas the teas made from the leaves and the green gumballs were not very resinous, having a taste reminiscent of pine needle tea, and lighter in color, though quite astringent.
Because the leaves and green gumball tea were so similar in terms of taste and aroma, we can guess that one could use them both in similar ways, even though we could not find uses traditional uses mention the green gumballs. Keep in mind though, that this is just our conjecture.
Additionally, the teas from the leaves and green gumballs were actually quite pleasant, so if we had to drink them for a case of respiratory infections, it wouldn’t be so bad!
Below you can see a photo from the experiment which shows the vary depth of color from the different teas!

Conclusion
In this article, we worked together to debunk the “DIY Tamiflu®” myth by digging deeper into the chemistry behind drug design and the ethnobotanical resources of the Americas and China. But, most of all, we learned that small phrases and briefly shared ideas can have enormous impacts on the decisions people make about their health. Evidence comes in many forms, some more reliable than others. Your job? To follow the evidence, not the claim.
Disclaimer: These statements have not been evaluated by the Food and Drug Administration. This information and these natural products have been presented for educational purposes only, and are not intended for use in the diagnosis, treatment, curing, or prevention of any disease. In addition, we make no claims about the efficacy of Tamiflu® in this article.
The authors of this article are not sponsored or supported by any pharmaceutical company nor any natural health/herb company and claim no conflicts of interest.

Sources
[1] Example of one of the many “DIY Tamiflu” claims, starting at minute 03:05 and see description, (YouTube–31,200 views): A wonderful medicial tree: Sweet Gum
[2] Sweetgum: An ancient source of beneficial compounds with modern benefits
[3] Liquidambar styraciflua: a renewable source of shikimic acid
[4] Tamiflu: Consumer Questions and Answers (FDA)
[5] Tamiflu Prescribing Information from Tamiflu.com
[7] 44 – Antiviral Drugs for Influenza and Other Respiratory Virus Infection
[8] Neuraminidase Inhibitors: Mechanism of Action (video)
[9] Neuraminidase Inhibitors for Influenza
[12] The Process of Structure-based Drug Design
[13] Khan Academy: Ligands & Receptors
[14] Enzymology: 3. Active Site & Regulation
[15] Figure 5: Structures of influenza virus sialidase with zanamivir and oseltamivir carboxylate.
[16] Structure of Shikimic Acid
[19] Oseltamivir total synthesis
[20] American Chemical Society: Production and Synthetic Modifications of Shikimic Acid
[21] Fallacy of Division
[24] Sweet Gum, Native American Ethnobotany Database
[25] USDA – Sweetgum Plant Profile
[26] A Reference Guide to Medicinal Plants (pp. 419-421)
[27] Traditional Sources List
- Resources of the Southern Fields and Forests
- Native American Ethnobotany DB
- The Family Herbal, 1812
- The Dispensatory of the United States of America, 1918 – Joseph P. Remington, Horatio C. Wood
- Specific Medication and SPecific Medicines, 1870 – Scudder
- A Manual of Organic Materia medica and Pharmacognosy, 1917 – Sayre
- King’s American Dispensatory 1898 – Felter, Lloyd
- King’s American Dispensatory 1898 – Felter, Lloyd
- The Eclectic Materia Medica, Pharmacology and Therapeutics, 1922 – Felter
- The Physiomedical Dispensatory, 1869 – Cook
[28] Liquidambar formosana Hance: A Mini-review of Chemical Constituents and Pharmacology
[29] Fructus liquidambar – Lulutong
[30] Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009)
[31] Enzyme Inhibition
[32] Role of Induced Fit in Enzyme Specificity: A Molecular Forward/Reverse Switch
[33] Chapter 15 – Viral surface glycoproteins in carbohydrate recognition: Structure and modelling
[34] Drug Design: Structure- and Ligand-Based Approaches
[35] A short overview on the medicinal chemistry of (-)-shikimic acid
[36] Mountain Medicine
[37] Chinese Herbal Medicine – Materia Medica, Portable 3rd Edition
[38] Lu Lu Tong Fructus Liquidambaris Beautiful Sweetgum Fruit
[40] Differences between in vitro, in vivo, and in silico studies
Appendix A
Disclaimer: Using extracts of these herbs is not a substitute for receiving proper medical attention. Seek out the help and advice of a licensed medical professional.
Please do not attempt to use extracts of these herbs to treat Influenza A and B on your own–and, remember that some of the compounds were being investigated for a bacterial neuraminidase, not a viral one. The extractions of the compounds discussed below are often far more complex than what we are capable of achieving at home, and their efficacy as neuraminidase inhibitors is still being investigated. The purpose of research like this is to provide novel compounds for the purpose of drug development.
BLACK ELDERBERRY — Swaminathan, K., Dyason, J.C., Maggioni, A. et al. Binding of a natural anthocyanin inhibitor to influenza neuraminidase by mass spectrometry. Anal Bioanal Chem 405, 6563–6572 (2013). https://doi.org/10.1007/s00216-013-7068-x Abstract: The binding of a natural anthocyanin to influenza neuraminidase has been studied employing mass spectrometry and molecular docking. Derived from a black elderberry extract, cyanidin-3-sambubiocide has been found to be a potent inhibitor of sialidase activity. This study reveals the molecular basis for its activity for the first time. The anthocyanin is shown by parallel experimental and computational approaches to bind in the so-called 430-cavity in the vicinity of neuraminidase residues 356–364 and 395–432. Since this antiviral compound binds remote from Asp 151 and Glu 119, two residues known to regulate neuraminidase resistance, it provides the potential for the development of a new class of antivirals against the influenza virus without this susceptibility. RHODIOLA ROSEA– Jeong HJ, Ryu YB, Park SJ, et al. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg Med Chem. 2009;17(19):6816-6823. doi:10.1016/j.bmc.2009.08.036 Abstract: Five flavonols (3, 5, and 9-11) were isolated from Rhodiola rosea, and compared with commercially available flavonoids (1, 2, 4, 6-8, and 12-14) to facilitate analysis of their structure-activity relationship (SAR). All compounds (1-14) showed neuraminidase inhibitory activities with IC(50) values ranging from 0.8 to 56.9 microM. The in vitro anti-influenza virus activities of flavonoids 1-6, 8-12, and 14 were evaluated using two influenza viral strains, H1N1 (A/PR/8/34) and H9N2 (A/Chicken/Korea/MS96/96), testing their ability to reduce virus-induced cytopathic effect (CPE) in MDCK cells. We found that the activity of these compounds ranged from 30.2 to 99.1 microM against H1N1- and 18.5 to 133.6 microM against H9N2-induced CPE. Of compounds 1-14, gossypetin (6) exhibited the most potent inhibitory activity, with IC(50) values of 0.8 and 2.6 microM on neuraminidases from Clostridium perfringens and recombinant influenza virus A (rvH1N1), respectively. In contrast, kaempferol (3) exhibited the highest activity against two influenza viruses, H1N1 and H9N2 with EC(50) values of 30.2 and 18.5 microM, respectively. Activity depended on the position and number of hydroxy groups on the flavonoids backbone. In kinetic studies, all isolated compounds behaved as noncompetitive inhibitors. CORYDALIS TURTSCHANINOVII — Kim JH, Ryu YB, Lee WS, Kim YH. Neuraminidase inhibitory activities of quaternary isoquinoline alkaloids from Corydalis turtschaninovii rhizome. Bioorg Med Chem. 2014;22(21):6047-6052. doi:10.1016/j.bmc.2014.09.004 Abstract: Clostridium perfringens is a Gram-positive spore-forming bacterium that causes food poisoning. The neuraminidase (NA) protein of C. perfringens plays a pivotal role in bacterial proliferation and is considered a novel antibacterial drug target. Based on screens for novel NA inhibitors, a 95% EtOH extract of Corydalis turtschaninovii rhizome showed NA inhibitory activity (68% at 30 μg/ml), which resulted in the isolation of 10 isoquinoline alkaloids; namely, palmatine (1), berberine (2), coptisine (3), pseudodehydrocorydaline (4), jatrorrhizine (5), dehydrocorybulbine (6), pseudocoptisine (7), glaucine (8), corydaline (9) and tetrahydrocoptisine (10). Interestingly, seven quaternary isoquinoline alkaloids 1-7 (IC50 = 12.8 ± 1.5 to 65.2 ± 4.5 μM) showed stronger NA inhibitory activity than the tertiary alkaloids 8-10. In addition, highly active compounds 1 and 2 showed reversible non-competitive behavior based on a kinetic study. Molecular docking simulations using the Autodock 4.2 software increased our understanding of receptor-ligand binding of these compounds. In addition, we demonstrated that compounds 1 and 2 suppressed bacterial growth. GLYCYRRHIZA GLABRA — Grienke U, Braun H, Seidel N, et al. Computer-guided approach to access the anti-influenza activity of licorice constituents. J Nat Prod. 2014;77(3):563-570. doi:10.1021/np400817j Abstract: Neuraminidase (NA), a key enzyme in viral replication, is the first-line drug target to combat influenza. On the basis of a shape-focused virtual screening, the roots of Glycyrrhiza glabra (licorice) were identified as plant species with an accumulation of constituents that show 3D similarities to known influenza NA inhibitors (NAIs). Phytochemical investigation revealed 12 constituents identified as (E)-1-[2,4-dihydroxy-3-(3-methyl-2-butenyl)phenyl]-3-(8-hydroxy-2,2-dimethyl-2H-1-benzopyran-6-yl)-2-propen-1-one (1), 3,4-dihydro-8,8-dimethyl-2H,8H-benzo[1,2-b:3,4-b’]dipyran-3-ol (2), biochanin B (3), glabrol (4), glabrone (5), hispaglabridin B (6), licoflavone B (7), licorice glycoside B (8), licorice glycoside E (9), liquiritigenin (10), liquiritin (11), and prunin (12). Eleven of these constituents showed significant influenza virus NA inhibition in a chemiluminescence (CL)-based assay. Additional tests, including (i) a cell-based cytopathic effect inhibition assay (general antiviral activity), (ii) the evaluation of cytotoxicity, (iii) the inhibition of the NA of Clostridium perfringens (CL- and fluorescence (FL)-based assay), and (iv) the determination of self-fluorescence and quenching, provided further perspective on their anti-influenza virus potential, revealing possible assay interference problems and false-positive results. Compounds 1, 3, 5, and 6 showed antiviral activity, most likely caused by the inhibition of NA. Of these, compounds 1, 3, and 6 were highly ranked in shape-focused virtual screening. SCUTELLARIA BAICALENSIS — Dou J, Chen L, Xu G, et al. Effects of baicalein on Sendai virus in vivo are linked to serum baicalin and its inhibition of hemagglutinin-neuraminidase. Arch Virol. 2011;156(5):793-801. doi:10.1007/s00705-011-0917-z Abstract: Parainfluenza viruses are significant respiratory-tract pathogens that are notorious for infecting children. However, there are no clinical drugs to control the infection caused by these viruses. Sendai virus (SeV) belongs to the family Paramyxoviridae and causes fatal pneumonia in mice, its natural host. Baicalein is a flavonoid derived from the root of Scutellaria baicalensis, which is a traditional Chinese medicine that has been used for hundreds of years and has demonstrated a variety of biological activities. Our findings reveal that oral administration of baicalein to mice infected with Sendai virus results in a significant reduction in virus titers in the lungs and protection from death. The in vivo inhibitory effects of baicalein on Sendai virus are determined by baicalin in the serum. The mean IC(50) of baicalin was 0.71 μg/ml in an HA inhibition assay and 3.22 μg/ml in an NA inhibition assay. The mean IC(50) of baicalin in a CPE assay was measured to be 0.70 μg/ml, and significant inhibition was observed in a plaque assay at a concentration of 1.6 μg/ml baicalin in overlay medium, which suggests that baicalein is a potential anti-parainfluenzaviral agent in vivo. ARRAY OF HERBS — Sahoo M, Jena L, Rath SN, Kumar S. Identification of Suitable Natural Inhibitor against Influenza A (H1N1) Neuraminidase Protein by Molecular Docking. Genomics Inform. 2016;14(3):96-103. doi:10.5808/GI.2016.14.3.96 Abstract: The influenza A (H1N1) virus, also known as swine flu is a leading cause of morbidity and mortality since 2009. There is a need to explore novel anti-viral drugs for overcoming the epidemics. Traditionally, different plant extracts of garlic, ginger, kalmegh, ajwain, green tea, turmeric, menthe, tulsi, etc. have been used as hopeful source of prevention and treatment of human influenza. The H1N1 virus contains an important glycoprotein, known as neuraminidase (NA) that is mainly responsible for initiation of viral infection and is essential for the life cycle of H1N1. It is responsible for sialic acid cleavage from glycans of the infected cell. We employed amino acid sequence of H1N1 NA to predict the tertiary structure using Phyre2 server and validated using ProCheck, ProSA, ProQ, and ERRAT server. Further, the modelled structure was docked with thirteen natural compounds of plant origin using AutoDock4.2. Most of the natural compounds showed effective inhibitory activity against H1N1 NA in binding condition. This study also highlights interaction of these natural inhibitors with amino residues of NA protein. Furthermore, among 13 natural compounds, theaflavin, found in green tea, was observed to inhibit H1N1 NA proteins strongly supported by lowest docking energy. Hence, it may be of interest to consider theaflavin for further in vitro and in vivo evaluation. |